Separation and complexation of palladium(ii) with a new soft N-donor ligand 6,6′-bis(5,6-dinonyl-1,2,4-triazin-3-yl)-2,2′-bipyridine (C9-BTBP) in nitric acid medium†
Abstract
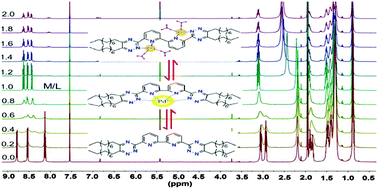
Soft N-donor bis-triazine ligands developed for the separation of minor actinides (MAs) from lanthanides have been intensively studied for the past two decades. However, the investigation on the recovery and complexation of fission products Pd(II) with these ligands has rarely been reported to date. Herein, the synthesis, and solvent extraction of Pd(II) and some typical metals from HNO3 solutions as well as Pd(II) complexation with a new soft N-donor ligand C9-BTBP were presented. The C9-BTBP ligand exhibited high extraction capability and high selectivity for Pd(II) in HNO3 solution. Both 1 : 1 and 2 : 1 Pd(II)–C9-BTBP complexes were found in solution by a combination of ESI-MS and 1H NMR titration experiments. A solid binuclear Pd(II) complex [Pd2(NO3)4·(C9-BTBP)]·2.5H2O was synthesized and characterized by elemental analysis, DSC-TGA and 1H NMR. This is the first example that a binuclear Pd(II) complex with any N-donor bis-triazine ligand has been confirmed both in solution and in the solid state.

 Please wait while we load your content...
Please wait while we load your content...