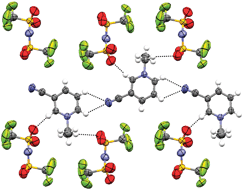
1-Alkyl-n-cyanopyridinium and 1-alkyl-n-(trifluoromethyl)pyridinium salts have been synthesised and characterised in order to compare the effects of different electron-withdrawing functional groups on their ability to form ionic liquids. The presence of the electron-withdrawing nitrile or trifluoromethyl substituent on the pyridinium ring leads to salts with higher melting points than with the corresponding 1-alkylpyridinium or 1-alkylpicolinium cations. Solid-state structures were determined by single crystal X-ray crystallography for seven salts; 1-methyl-4-cyanopyridinium methylsulfate, and 1-methyl-3-cyanopyridinium, 1-methyl-4-cyanopyridinium, 1-ethyl-2-cyanopyridinium, 1-ethyl-3-cyanopyridinium, 1-ethyl-4-cyanopyridinium and 1-ethyl-4-(trifluormethyl)pyridinium bis{(trifluoromethyl)sulfonyl}imide, and show the effects of ring-substitution position on hydrogen-bonding in the solid-state and on melting points.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...