From dinuclear to tetranuclear zinc complexes through carboxylate donors: structural and luminescence studies†
Abstract
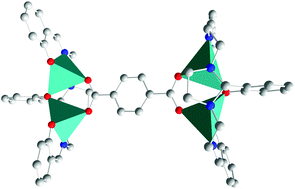
Carboxylate zinc complexes of general formula [Zn2Lx(p-OOC–C6H4–CHO)], [Zn2Lx(o-OOC–C6H4–COOH)], [(Zn2Lx)2(OOC–CH2–COO)], [(Zn2Lx)2(OOC–CH2CH2–COO)] and [(Zn2Lx)2(p-OOC–C6H4–COO)] (x = 1, 2; H3L1 = 2-(2-hydroxyphenyl)-1,3-bis[4-(2-hydroxyphenyl)-3-azabut-3-enyl]-1,3-imidazolidine and H3L2 = 2-(5-bromo-2-hydroxyphenyl)-1,3-bis[4-(5-bromo-2-hydroxyphenyl)-3-azabut-3-enyl]-1,3-imidazolidine) were isolated with different solvates. The crystal structures of [Zn2L1(p-OOC–C6H4–CHO)]·2.5DMSO·2H2O, [(Zn2L1)2(OOC–CH2CH2–COO)]·4.25H2O·0.75MeOH·0.5MeCN, [(Zn2L2)2(OOC–CH2CH2–COO)]·7H2O·0.25MeOH and [(Zn2L1)2(p-OOC–C6H4–COO)]·2.5H2O·MeOH·6EtOH demonstrate that their different nuclearities are a function of the carboxylate donor, with the

 Please wait while we load your content...
Please wait while we load your content...