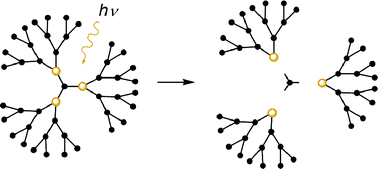
Two dendrimer cores, 1a and 2a, that contain o-nitrobenzyl photolabile moieties, lack hydrolytically sensitive ester linkages and possess three and six sites for dendron attachment, respectively, have been alkylated to provide methylated core analogs 1b and 2b as well as second-generation benzylaryl ether dendrimer 1c and third-generation dendrimer 2c. These dendrimers undergo clean photocleavage as indicated by the evolution of isosbestic points in the UV spectra during photolysis. In addition, the nature of the photodegradation products was confirmed by observing the photolyses by both 1H NMR and GPC.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...