3-Formylchromone based topoisomerase IIα inhibitors: discovery of potent leads†
Abstract
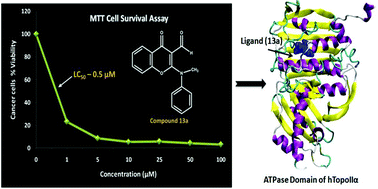
Substituted 3-formylchromones were synthesized and evaluated as inhibitors of the human DNA topoisomerase IIα (hTopo-IIα) enzyme. The results of the decatenation, relaxation and DNA intercalation assays revealed that the compounds (11b, 12a, 12b, 12d, 12e, 13a and 13b) exhibited potent inhibitory activity against the hTopo-IIα enzyme, and are nonintercalating agents. These compounds also possess significant in vitro cytotoxicity (LC50 ranges from 0.5–8.6 μM) against prostate (PC-3) cancerous cell line as seen in comparison to the standard drug etoposide. To further probe the plausible mode of action of 3-formylchromone derivatives, molecular docking studies have also been carried out, which showed that the compounds under investigation fitted well in the ATP binding pocket of hTopo-IIα enzyme with good docking scores and form nonbonding interactions with the crucial residues of the catalytic site.

 Please wait while we load your content...
Please wait while we load your content...