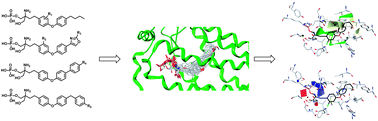
Spingosine-1-phosphate receptor 1 (S1P1) has been actively pursued as an important therapeutic target in immune regulation. A series of 2-substituted 2-aminopropane-1,3-diols were designed and synthesized as selective S1P1 agonists. Most of the compounds with a biphenyl ether scaffold showed moderate to excellent S1P1/S1P3 selectivity. Compound 40c is identified as a potent S1P1 agonist with 350-fold S1P1/S1P3 selectivity. 39c, the alcohol form of 40c exerted good lymphopenia activity in vivo but with weak influence on heart rate. To investigate the SARs of 2-substituted 2-aminopropane-1,3-diols in more details, COMFA (q2 = 0.547, r2 = 0.986) and COMSIA (q2 = 0.544, r2 = 0.943) models were established based on molecular docking alignment, which were validated with high reliability in predicting activities of agonists. The 3D-QSAR models will be helpful in the design of novel, potent and selective S1P1 agonists.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...