Engineering the synthetic potential of β-lactam synthetase and the importance of catalytic loop dynamics†‡
Abstract
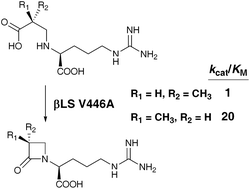
The 2-azetidinone ring of the Class A and D β-lactamase inhibitor clavulanic acid (1) is synthesized by the ATP-utilizing enzyme β-lactam synthetase (βLS). A hydroxyethyl group attached to C-6 of 1 in the (S) configuration markedly enhances the efficacy of this compound against Class C β-lactamases. Guided by a series of X-ray structures of βLS, we have engineered this enzyme to act upon a methylated substrate analogue to give selectively the (3S)-methyl β-lactam core, which, upon closure of the second ring of the bicyclic system of 1, would lead to the (6S)-methylated clavulanic acid derivative.

 Please wait while we load your content...
Please wait while we load your content...