Syntheses and properties of trimethylaminophenoxy-substituted Zn(ii)-phthalocyanines†
Abstract
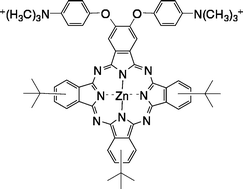
The syntheses, photophysical properties and in vitro biological behavior of a series of nine Zn(II)-phthalocyanines (ZnPcs) bearing one to eight positively-charged trimethylaminophenoxy groups are reported. All ZnPcs are highly soluble in polar organic

 Please wait while we load your content...
Please wait while we load your content...