SAR mining and its application to the design of TRPA1 antagonists
Abstract
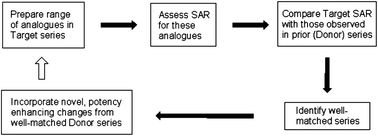
Given the large amounts of screening data now available, empirical methods derived from matched-molecular pairs are being used as a means for suggesting bioisosteric replacements to the medicinal chemist. The pairwise analysis of compounds has been extended to the pairwise analysis of series to bring further context to these suggestions. A validation dataset derived from recent literature has been used to demonstrate that, given a series of active compounds, this approach would be expected to predict a more potent compound, if it exists, in around 46% of cases. The approach has been successfully applied to a series of TRPA1

 Please wait while we load your content...
Please wait while we load your content...