Dual-channel bipolar electrode focusing: simultaneous separation and enrichment of both anions and cations†
Abstract
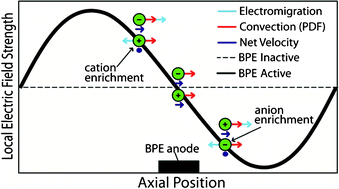
In this paper we show that a microelectrochemical cell comprising two parallel microchannels spanned by a single bipolar electrode can be used to simultaneously enrich and separate both anions and cations within a single microchannel. This is possible because reduction and oxidation of water at the cathodic and anodic poles of the bipolar electrode, respectively, lead to ion depletion zones. Specifically, TrisH+ is neutralized by OH− at the cathodic pole, while acetate buffer is neutralized by H+ at the anodic pole. This action creates a local electric field gradient having both positive and negative components, and hence positive and negative ions follow their respective field gradients leading to separation. In the presence of an opposing counter-flow (pressure driven flow in this case), enrichment also occurs. In addition to separation and enrichment in a single channel, it is also possible to simultaneously enrich cations in one microchannel and anions in the other. Enrichment is achieved by controlling experimental parameters, including the type of buffer and the direction and magnitude of the opposing counter-flow.

 Please wait while we load your content...
Please wait while we load your content...