Organic modification of the interlayer surface of kaolinite with propanediols by transesterification†
Abstract
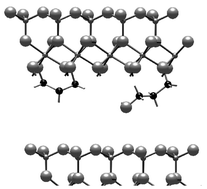
Propanediols (1,2- and 1,3-propanediols) were reacted with methoxy-modified kaolinite to form hydroxypropoxy-modified kaolinites by transesterification. Grafting occurred on the AlOH groups of the interlayer surface, which provides unique asymmetrical interlayer environments surrounded by both hydroxy groups of the diols and silicate layers, suggesting potential applications for specific adsorption and regioselective reactions in the interlayer spaces. When the reaction time was increased, 1,3-propanediol partly forms a bridge type grafting where both OH groups were bonded. Methoxy groups on the interlayer surface of kaolinite were eliminated by the grafting. The amounts of the grafted alkoxy groups per Al2Si2O5(OH)4 unit were larger than that of methoxy-modified kaolinite, indicating that not only transesterification but also further grafting onto the AlOH groups proceeded. Under milder conditions, 1,2- and 1,3-propanediols were not grafted onto the interlayer surface, but molecularly intercalated.

 Please wait while we load your content...
Please wait while we load your content...