Utilizing hydrogen underpotential deposition in CO reduction for highly selective formaldehyde production under ambient conditions†
Abstract
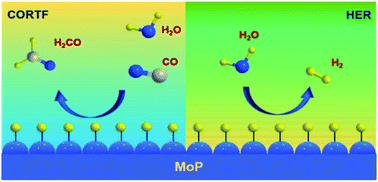
Formaldehyde is an essential building block for hundreds of chemicals and a promising liquid organic hydrogen carrier (LOHC), yet its indirect energy-intensive synthesis process prohibits it from playing a more significant role. Here we report a direct CO reduction to formaldehyde (CORTF) process that utilizes hydrogen underpotential deposition to overcome the thermodynamic barrier and the scaling relationship restriction. This is the first time that this reaction has been realized under ambient conditions. Using molybdenum phosphide as a catalyst, formaldehyde was produced with nearly a 100% faradaic efficiency in aqueous KOH solution, with its formation rate being one order of magnitude higher compared with the state-of-the-art thermal catalysis approach. Simultaneous tuning of the current density and reaction temperature led to a more selective and productive formaldehyde synthesis, indicating the electrochemical and thermal duality of this reaction. DFT calculations revealed that the desorption of the *H2CO intermediate likely served as the rate-limiting step, and the participation of H2O made the reaction thermodynamically favorable. Furthermore, a full-cell reaction set-up was demonstrated with CO hydrogenation to HCHO achieved without any energy input, which fully realized the spontaneous potential of the reaction. Our study shows the feasibility of combining thermal and electrochemical approaches for realizing the thermodynamics and for scaling relationship-confined reactions, which could serve as a new strategy in future reaction design.


 Please wait while we load your content...
Please wait while we load your content...
