Poly(N-vinylpyrrolidone)–H2O2 and poly(4-vinylpyridine)–H2O2 complexes: solid H2O2 equivalents for selective oxidation of sulfides to sulfoxides and ketones to gem-dihydroperoxides†
Abstract
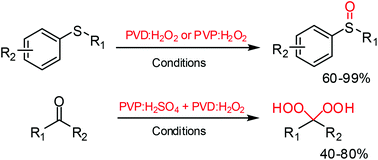
Complexes of poly(N-vinylpyrrolidone) (PVD) and poly(4-vinylpyridine) (PVP) with hydrogen peroxide have been prepared and their synthetic utility as solid H2O2 equivalents for the selective oxidation of sulfides to sulfoxides and ketones to gem-dihydroperoxides has been studied. These complexes are convenient and safe alternatives to H2O2 solutions and it is found that various symmetric as well as unsymmetrical sulfides undergo oxidation under mild conditions to provide the respective sulfoxides in high yields. A series of gem-dihydroperoxides were obtained from the corresponding ketones in good yields under ambient conditions.

 Please wait while we load your content...
Please wait while we load your content...