Selective oxidation of methanol to methyl formate on catalysts of Au–Ag alloy nanoparticles supported on titania under UV irradiation†
Abstract
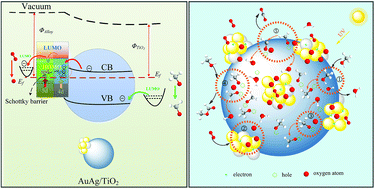
We find that the Au–Ag alloy nanoparticles supported on titania exhibit superior methanol conversion and methyl formate selectivity for selective oxidation of methanol by low partial pressure oxygen in air under UV irradiation in the 15 °C–45 °C temperature range, with the highest methanol conversion above 90% and the highest selectivity towards methyl formate above 85%. The only by-product definitely detected is CO2. The superior photocatalytic performance of the catalyst is closely related to the special structure of the catalyst and the electronic properties of the alloy, which reduce the recombination of the photo-excited electron–hole pairs by transferring the photo-excited electrons in time from the conduction band of titania to the alloy on the one hand, and elevate the negative charge level of the alloy surface by the spd hybridization, the formation of Schottky barriers, the electron transfer from the conduction band of titania to the metal as well as the interband and intraband electron transitions under UV irradiation on the other hand. The photo-generated holes are responsible for the oxidation from methanol to coordinated methoxy, from coordinated methoxy to coordinated formaldehyde and finally to carbon dioxide. The methyl formate selectivity is dependent on the density of the surface methoxy. To enhance the efficiency of electron–hole separation is beneficial to the formation of the coordinated methoxy and coordinated formaldehyde and thus the selectivity to methyl formate. The negative charges on the surface of the metal are responsible for the dissociation of oxygen, which is the rate-determining step in the reaction. The dissociative oxygen repels the water molecules formed from the surface hydroxyls and refills the oxygen vacancies on the surface of titania. The surface oxygen is the acceptor of the hydrogen dissociated from methanol and/or methoxy and thus is beneficial for the formation of the coordinated methoxy and coordinated formaldehyde. The oxygen partial pressure remarkably influences the methanol conversion and the methyl formate selectivity. The light intensity has a remarkable impact on the methanol conversion but not on the methyl formate selectivity. These findings provide useful insight into the design of catalysts for selective oxidation of methanol to methyl formate in a more green way.

 Please wait while we load your content...
Please wait while we load your content...