Efficient synthesis of quinazoline-2,4(1H,3H)-diones from CO2 using ionic liquids as a dual solvent–catalyst at atmospheric pressure†
Abstract
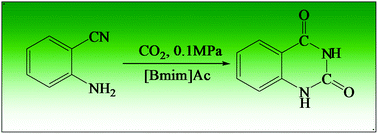
The highly efficient transformation of CO2 into value-added chemicals is an interesting topic in green chemistry. In this work, we studied the synthesis of quinazoline-2,4(1H,3H)-diones from CO2 and 2-aminobenzonitriles in a series of ionic liquids (ILs). It was found that 1-butyl-3-methylimidazolium acetate ([Bmim]Ac), a simple and easily prepared IL, could act as both solvent and catalyst, the reactions could be carried out very efficiently at atmospheric pressure of CO2, and a high yield of the products was obtained. Further study indicated that the IL was also very efficient for converting other 2-aminobenzonitriles into their corresponding quinazoline-2,4(1H,3H)-diones in high yields at atmospheric pressure. Moreover, the separation of the products from the IL was very easy, and the IL could be reused at least five times without considerable loss in catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...