Spectroscopic and electrochemical characterization of heteropoly acids for their optimized application in selective biomass oxidation to formic acid†
Abstract
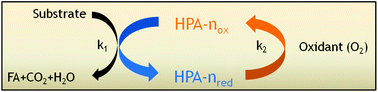
Different Keggin-type polyoxometalates have been synthesized and characterized in order to identify optimized homogeneous catalysts for the selective oxidation of biomass to formic acid (FA) using oxygen as an oxidant and p-toluenesulfonic acid as an additive. Applying the optimized polyoxometalate catalyst system H8[PV5Mo7O40] (HPA-5), a total FA-yield (with respect to carbon in the biogenic feedstock) of 60% for glucose within 8 h reaction time and 28% for cellulose within 24 h reaction time could be achieved. The transformation is characterized by its mild reaction temperature, its excellent selectivity to FA in the liquid product phase and its applicability to a very wide range of biogenic raw materials including non-edible biopolymers and complex biogenic mixtures.

 Please wait while we load your content...
Please wait while we load your content...