Evaluating aqueous flow battery electrolytes: a coordinated approach†
Abstract
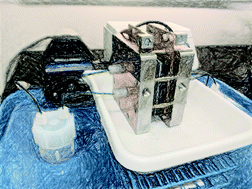
Here, we outline some basic pitfalls in the electrochemical investigation of aqueous metal complexes and advocate for the use of bulk electrolysis in redox flow cells for electrolyte analysis. We demonstrate the methods of operation and performance of a lab scale redox flow battery (RFB), which is assembled from unmodified, commercially available material and cycled with a vanadium electrolyte in order to provide a comparative baseline of expected performance. Common misconceptions about the thermodynamic window for water splitting are addressed and further express the need to develop next-generation aqueous redox flow battery electrolytes. Although non-aqueous electrolytes are a popular approach, they suffer from distinct challenges that limit energy and power density in comparison with aqueous electrolytes. Expanding the scope of aqueous electrolytes to include metal–chelate complexes allows electrolytes to be as tailorable as organic species, while maintaining robust metal-based redox processes. A flow battery assembly and operation guide is provided to help facilitate the use of flow battery testing in the evaluation of next-generation electrolytes.
- This article is part of the themed collections: 2020 Frontier and Perspective articles and New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...
