Bis(pentafluorophenyl)phenothiazylborane – an intramolecular frustrated Lewis pair catalyst for stannane dehydrocoupling†
Abstract
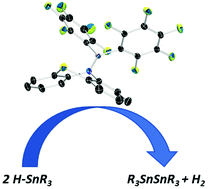
We synthesized a novel Lewis acidic aminoborane containing a phenothiazyl substituent and demonstrated its potential to catalytically promote the dehydrocoupling of tin hydrides. The observed reactivity would imply a homolytic frustrated Lewis pair type mechanism, however computational analysis suggests a heterolytic mechanism for this reaction. This result represents one of the first frustrated Lewis pair systems to dehydrocouple stannanes in a heterolytic fashion.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...