Barium-promoted hydrothermal stability of monolithic Cu/BEA catalyst for NH3-SCR†
Abstract
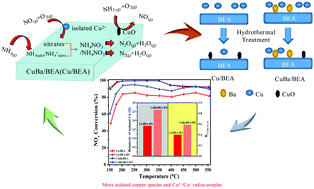
As a promising candidate for NOx elimination from the emission of diesel engines, the enhancement effect of Ba on the hydrothermal stability of cordierite supported CuBa/BEA at 600 °C for 48 h was investigated by XRD, NH3-TPD, H2-TPR, XPS, EPR, TEM and in situ DRIFTS. Different properties and amounts of active species are significant factors contributing to the enhanced hydrothermal stability of CuBa/BEA-HT. CuBa/BEA-HT has more Cu2+/Cu+ redox-couples and stronger interactions than Cu/BEA-HT, indicating an excellent redox property of the active species in CuBa/BEA-HT. The better redox property of CuBa/BEA-HT produces more nitrates that easily participate in the NH3-SCR reaction, which enhances the low-temperature activity. Furthermore, as observed from EPR and H2-TPR, the appearance of more isolated Cu2+ species and fewer CuO species also contribute to the higher hydrothermal stability of CuBa/BEA-HT.


 Please wait while we load your content...
Please wait while we load your content...