Cobalt complexes with hemilabile o-iminobenzoquinonate ligands: a novel example of redox-induced electron transfer†
Abstract
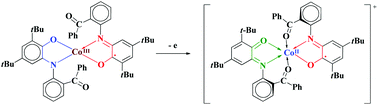
The tetracoordinated square-planar CoIII complex (imSQC(O)Ph)CoIII(APC(O)Ph) (1) bearing a radical anion and the closed-shell o-amidophenolate forms of the functionalized o-aminophenol H2LC(O)Ph were synthesized. The intermediate spin state (SCo = 1) CoIII center was found for compound 1. The cyclic voltammogram of derivative 1 contains two oxidative processes and one reductive redox process as well as an additional multi-electron wave at high negative potentials above −2 V, which can involve both the ligand and metal center. One-electron oxidation of 1 by silver triflate produces the [(imSQC(O)Ph)CoII(imQC(O)Ph)]OTf·2toluene (2) derivative with the trigonal prismatic coordination environment of the metal arising from the additional coordination of –C(O)Ph hemilabile groups. This is a first example of a trigonal prismatic coordination polyhedron in cobalt-based complexes featuring o-iminobenzoquinone ligands. The trigonal prismatic geometry achieved by the unique flexibility of the ligand allows metal-to-ligand redox-induced electron transfer (RIET). Chemical oxidation of complex 1 promotes the reduction of CoIII to CoII in compound 2 due to the redox-active nature of o-iminobenzoquinonate ligands. Remarkably, this is the first example of RIET in cobalt-based derivatives with this type of ligand. The oxidative states of the ligands and cobalt ion in both complexes were unequivocally established according to the X-ray data collection by using the utility of “metric oxidation state” (MOS). The spin states of the metal centers were unambiguously determined by density functional theory. The strong antiferromagnetic exchange via metal–ligand interactions is dominant in compounds 1 and 2, giving the doublet (S = 1/2) and triplet (S = 1) ground spin state, respectively.


 Please wait while we load your content...
Please wait while we load your content...