A ligand substituted tungsten iodide cluster: luminescence vs. singlet oxygen production†
Abstract
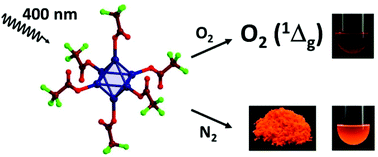
Octahedral tungsten iodide clusters equipped with apical ligands (L) are synthesized to implement substantial photophysical properties. The [W6I8(CF3COO)6]2− cluster reported herein is the first example of a family of ligand substituted [W6I8L6]2− clusters. Such compounds are expected to exhibit a rich photochemistry in which the apical ligands play a crucial role. The versatile solid state and solution phase photophysical properties of (TBA)2[W6I8(CF3COO)6] described herein parallel characteristics obtained in some photophysically active organic compounds, including a broad absorption in the UV/VIS region. Upon irradiation of this compound, a broad red emission is observed in the VIS/NIR region resulting from excited triplet states, and singlet oxygen (a1Δg) is generated in the presence of O2.

 Please wait while we load your content...
Please wait while we load your content...