Dinuclear clathrochelate complexes with pendent cyano groups as metalloligands†
Abstract
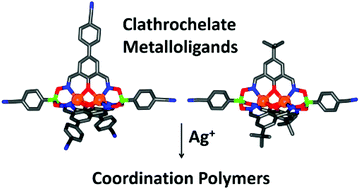
Dinuclear clathrochelate complexes with two, three, four, or five cyano groups in the ligand periphery were prepared following two distinct synthetic strategies: (a) zinc(II)- or cobalt(II)-templated polycondensation reactions of CN-functionalized arylboronic acids and phenoldioximes, or (b) postsynthetic cross-coupling reactions of polybrominated zinc(II) clathrochelates with 4-cyanophenylboronic acid. The new clathrochelate complexes were used as metalloligands for the construction of heterometallic Zn2+/Ag+ and Co2+/Ag+ coordination polymers (CPs), which were characterized by single crystal X-ray diffraction and FT-IR. A one-dimensional CP was observed for ditopic clathrochelates, whereas two- and three-dimensional CPs were generated from tetra- and pentatopic metalloligands. The three-dimensional network is unique as it displays an unprecedented network topology with the point symbol (8·102)(82·104)(82·10)(83·103). Furthermore, it is a self-catenated net with an extremely high topological density.


 Please wait while we load your content...
Please wait while we load your content...