Syntheses, structures and anti-tumor activity of four new organotin(iv) carboxylates based on 2-thienylselenoacetic acid†
Abstract
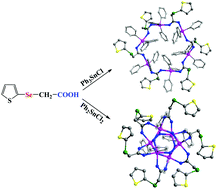
With the 2-thienylselenoacetic acid ligand, four new organotin complexes, [Me3Sn(O2CCH2SeC4H3S-o)]n (1), [(Ph3Sn)6(O2CCH2SeC4H3S-o)6] (2), [(Me2Sn)4(μ3-O)2(O2CCH2SeC4H3S-o)4] (3), and [(PhSn)6(μ3-O)6(O2CCH2SeC4H3S-o)6] (4), have been synthesized and characterized by X-ray crystallography, elemental analysis, FT-IR and NMR (1H, 13C, and 119Sn) spectroscopy. The structure analysis indicates that complex 1 adopts a 1D infinite zig-zag chain structure, while complex 2 shows a centrosymmetric hexanuclear 24-membered macrocycle. In contrast, complex 3 and complex 4 display ladder and drum structures, respectively. Examination of the non-covalent intermolecular contacts in complex 3 reveals the existence of the C–H⋯O and C–H⋯π interactions, which play an important function in the supramolecular construction. These compounds are rare examples of selenium carboxylic acid-based organotin derivatives. Furthermore, the anti-tumor activity of complexes 1–3 has also been studied. Importantly, the anti-proliferative properties and possible mechanism of complex 2 are preliminarily investigated. The results demonstrate that complex 2 could induce apoptotic cell death via accumulation of ROS and collapse of the mitochondrial membrane permeabilization (MMP).

 Please wait while we load your content...
Please wait while we load your content...