Surface functionalization of dinuclear clathrochelates via Pd-catalyzed cross-coupling reactions: facile synthesis of polypyridyl metalloligands†
Abstract
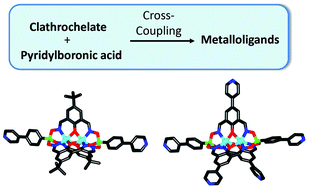
Dinuclear clathrochelate complexes are easily accessible by reaction of zinc(II) triflate or cobalt(II) nitrate with arylboronic acids and phenoldioximes. The utilization of brominated arylboronic acids and/or brominated phenoldioximes allows preparing clathrochelates with two, three, five or seven bromine atoms on the outside. These clathrochelates can undergo Pd-catalyzed cross-coupling reactions with 3- and 4-pyridylboronic acid to give new metalloligands featuring up to seven pyridyl groups. The pyridyl-capped clathrochelates display characteristics which make them interesting building blocks for structural supramolecular chemistry: they are rigid, large (up to 2.7 nm), luminescent (for M = Zn), and anionic. The pentatopic pyridyl ligands display an unusual trigonal bipyramidal geometry.

 Please wait while we load your content...
Please wait while we load your content...