Cyclometalation and coupling of a rigid 4,5-bis(imino)acridanide pincer ligand on yttrium†
Abstract
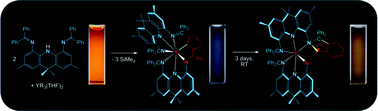
An extremely rigid NNN-donor proligand, 4,5-bis{(diphenylmethylene)amino}-2,7,9,9-tetramethylacridan, H[AIm2] was prepared in five steps starting from 5-methyl-2-aminobenzoic acid and 4-bromotoluene. Reaction of intensely orange H[AIm2] with LiCH2SiMe3 formed deep blue Lix[AIm2]x (x = 2 in the solid state), while reaction with [Y(CH2SiMe3)3(THF)2] (0.5 equiv.) afforded deep blue [Y(AIm2)(AIm′2)] (1; AIm′2 = an AIm2 ligand cyclometalated at the ortho-position of one of the phenyl rings). Compound 1 slowly isomerizes to form green-brown 2, which contains a single trianionic, hexadentate ligand that features one amine, two imine, and three amido donors. The acridanide backbone and one imine group in each of the original AIm2 ligands is intact, but the two acridanide backbones are now linked by an isoindoline heterocycle. Yttrium in 2 is coordinated to six nitrogen donors and the ortho carbon of an isoindoline phenyl substituent. The intense colours of H[AIm2], Lix[AIm2]x and 1 were shown by TD-DFT calculations to arise from charge transfer transitions from the HOMO, which is localized on the acridanide ligand backbone, to the LUMO and LUMO+1, which are localized on the imine substituents. The conversion of 1 to 2 was studied by UV-Visible absorption spectroscopy and is first-order with a half-life of 7.8 hours at room temperature.

 Please wait while we load your content...
Please wait while we load your content...