Investigations on the catalytic carboxylation of olefins with CO2 towards α,β-unsaturated carboxylic acid salts: characterization of intermediates and ligands as well as substrate effects†
Abstract
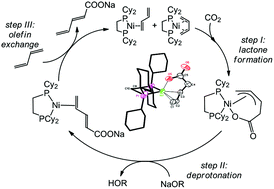
The carboxylation of olefins beyond ethylene towards α,β-unsaturated carboxylic acid salts and a detailed investigation on the critical steps in the catalysis are reported. The influence of two chelating phosphine ligands and several olefins on the elemental steps of the catalysis is shown. The work focusses on the formation of intermediate olefin complexes, lactone formation and base induced elimination of the lactone. The direct carboxylation of olefins is possible using nickel catalysts, which opens a new route towards the desired α,β-unsaturated carboxylic acid salts. The reaction works particularly well for 1,3-dienes and proceeds via the formation of allyl-carboxylates. The ability to form such allyl-type lactone complexes seems in this case to be the most challenging step towards satisfactory turnover numbers.

 Please wait while we load your content...
Please wait while we load your content...