Continuous expansion of the interplanar spacing of octacalcium phosphate by incorporation of dicarboxylate ions with a side chain
Abstract
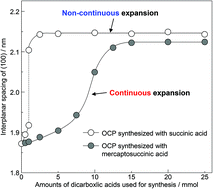
Octacalcium phosphate (OCP) is composed of apatitic and hydrated layers, and can incorporate dicarboxylate ions in its hydrated layers by substitution of HPO42−. The (100) interplanar spacing of OCP is increased by incorporation of dicarboxylate ions. Herein, we report continuous expansion of the interplanar spacing of OCP by incorporation of dicarboxylate ions with a side chain. We synthesized OCP with incorporated succinic acid (Suc) and mercaptosuccinic acid (Msuc) by a wet chemical process. The (100) interplanar spacing of OCP synthesized with Suc increased non-continuously as the amount of Suc used in the synthesis increased. In contrast, the (100) interplanar spacing of OCP synthesized with Msuc increased continuously with the amount of Msuc used during synthesis, and thus in the OCP. The values of the (100) interplanar spacing of OCP synthesized with Msuc first increased and then became constant as the amount of Msuc increased. OCP with incorporated Msuc formed a continuous solid solution. The expansion of the (100) interplanar spacing of OCP with incorporated Suc and Msuc depended on the chemical structure of the incorporated dicarboxylate ions. The only structural difference between Suc and Msuc is a mercapto (R–SH) side chain. Therefore, continuous solid solution formation was most likely induced by the R–SH side chain of Msuc. These results suggest that incorporation of dicarboxylic acids with a side chain is one approach to obtain OCP with arbitrary interlayer distance.

 Please wait while we load your content...
Please wait while we load your content...