New iron tetrazolate frameworks: synthesis, temperature effect, thermal behaviour, Mössbauer and magnetic studies†
Abstract
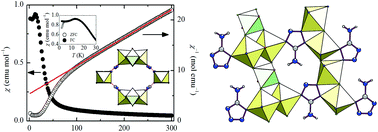
The exploration of the FeF3/FeF2-Hamtetraz-HF system in dimethylformamide by solvothermal synthesis evidences two isostructural 3D hybrid fluoroferrates. They are prepared from the same starting mixture at two different synthesis temperatures: 120 °C for [Hdma]·(Fe4IIFeIIIF8(H2O)2(amtetraz)4) (1) and 140 °C for [Hdma]1.5·(Fe4.5IIFe0.5IIIF7(H2O)(HCOO)(amtetraz)4) (2). Both compounds are characterized by single crystal X-ray diffraction, X-ray thermodiffraction, TGA analysis, Mössbauer spectrometry and SQUID magnetometry. They crystallize in the monoclinic system and are built from two distinct chains connected by aminotetrazolate anions. The first chain ∞(FeIIFN4) is common to 1 and 2 and can be found in numerous fluorides. In the second chain ∞(Fe3X12) (X = F, N, O), iron cations adopt both valence states Fe(II)/Fe(III). The hydrolysis of DMF implies the formation of a [Hdma]+ cation and a (HCOO)− anion. The presence of Fe3+ in both phases is evidenced by 57Fe Mössbauer spectrometry. The magnetic properties are studied and two transitions from a paramagnetic regime to a long range ordered state below 30 K and 5 K are identified.

 Please wait while we load your content...
Please wait while we load your content...