The synthesis and pH-dependent behaviour of Re(CO)3 conjugates with diimine phenolic ligands†
Abstract
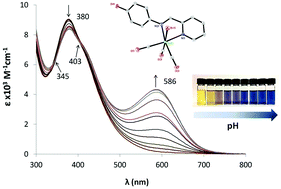
In this report we present a study of a series of Re(CO)3 pyridine–imine complexes with pendant phenol groups. We investigated the effects of the position of the

 Please wait while we load your content...
Please wait while we load your content...