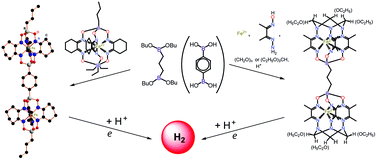
Iron(II) α-oximehydrazonate and α-dioximate bis-clathrochelates with apical hydrocarbon linkers were obtained by template condensation on an iron(II) ion followed by H+-catalyzed macrobicyclization of the bis-semiclathrochelate precursor with formaldehyde and triethyl orthoformate, and by transmetallation of the triethylantimony-containing clathrochelate precursor with diboron-containing bifunctional Lewis acids, respectively. The geometry of the para-phenylenediboron-capped iron(II) bis-clathrochelate studied by single-crystal X-ray diffraction is intermediate between a trigonal prism and a trigonal antiprism with a distortion angle of 20.4°; the rigidity of its C6H4 linker results in the presence of the expected three-fold pseudo-rotational B⋯Fe⋯B⋯B⋯Fe⋯B axis and a staggered conformation of the cyclohexane-containing chelate moieties. The cyclic voltammograms (CVs) for the oximehydrazonate bis-clathrochelates contain single one-electron (for each metallocentre, and therefore, two electrons per molecule) quasi-reversible reduction waves assigned to the redox-processes of Fe2+/+, and no interaction is observed between the two encapsulated iron(I)-containing metallocenters; six strong electron-withdrawing ethoxy substituents in the 1,3,5-triazacyclohexane capping fragments substantially affect the potential of this reduction. The corresponding waves for the dioximate complexes are irreversible: due to the structural rigidity of the caging tris-dioximate ligands, their reduced dianionic forms are unstable on the CV time scale. The CV for the hexaethoxy bis-clathrochelate complex contains one two-electron reversible oxidation wave assigned to the metal-centered oxidation of Fe2+/3+, whereas those for its dioximate analogs are quasi-reversible. The relative lability of the ligand cavity in binuclear oximehydrazonates causes a stabilization of both the oxidized and the reduced forms; the reduced iron(I)-containing species are highly electrocatalytically active in the hydrogen-producing 2H+/H2 reaction. Their higher activity as compared with that for dioximate bis-clathrochelates was explained by the higher availability of the catalytically active metallocentres for H+ ions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...