Ligand controlled dioxygen oxidation of rhenium nitrosyl complexes†
Abstract
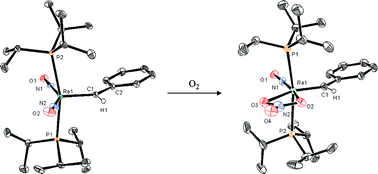
The addition of an excess of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(C6H5)}(NO)2(PR3)2][BArF4] (2a and 2b) in good yields. The treatment of 2b with dioxygen resulted in the oxidation of one of the nitrosyl
CH(C6H5)}(NO)2(PR3)2][BArF4] (2a and 2b) in good yields. The treatment of 2b with dioxygen resulted in the oxidation of one of the nitrosyl ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH{C6H5})(NO)2(PMe3)2]n+ (n = 1 and 2).
CH{C6H5})(NO)2(PMe3)2]n+ (n = 1 and 2).

 Please wait while we load your content...
Please wait while we load your content...