Dinuclear N-heterocyclic carbene complexes of silver(i), derived from imidazolium-linked cyclophanes
Abstract
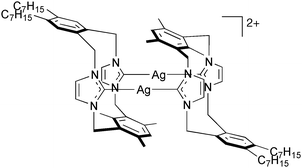
The synthesis of four new silver complexes of bidentate N-heterocyclic carbenes, derived from imidazolium-linked cyclophanes, has been achieved via a simple complexation reaction of the imidazolium-linked cyclophanes with the basic metal source Ag2O. The cyclophane structures contain two imidazolyl links between ortho-, meta- and mixed ortho/meta-substituted aromatic rings. The new silver carbene systems are thermally stable and have been characterised by NMR spectroscopy and X-ray crystallography. Three of the complexes have a dimeric structure of the form [L2Ag2]2+ in the solid state that is rigid on the NMR timescale in solution. The fourth complex has a neutral structure of the form [L(AgBr)2], the NMR studies suggesting some lability of the L–Ag bonding in solution.

 Please wait while we load your content...
Please wait while we load your content...