Strong π-acceptor sulfonated phosphines in biphasic rhodium-catalyzed hydroformylation of polar alkenes†
Abstract
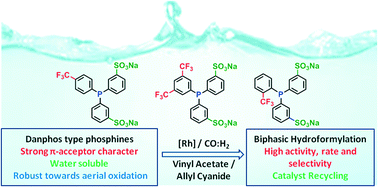
A new series of sulfonated triarylphosphines with a strong π-acceptor character were synthesized by direct sulfonation of trifluoromethylated neutral phosphines. Due to the deactivating character of the trifluoromethyl group, high oleum concentration and the use of boric acid to prevent phosphine oxidation were required for the sulfonation step. The new sulfonated phosphines are water-soluble and more inert toward oxidation than classical sulfonated phosphines. The use of these trifluoromethylated and sulfonated phosphines as ligands in the biphasic hydroformylation of vinyl acetate and allyl cyanide increases the rate of the reaction up to 4 times, compared to the results obtained with the non-trifluoromethylated counterparts, TPPMS, TPPDS and TPPTS. Moreover, it is possible to recycle the catalyst without a significant loss of the system activity.

 Please wait while we load your content...
Please wait while we load your content...