The outstanding performance of LDH-derived mixed oxide Mn/CoAlOx for Hg0 oxidation
Abstract
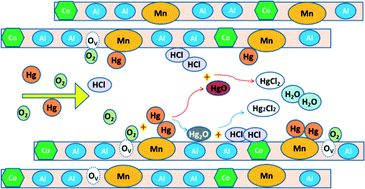
Layered double hydroxide (LDH)-derived Mn/CoAlOx catalysts prepared by coprecipitation showed excellent performance in the oxidation of elemental mercury (Hg0). The Hg0 conversion on 1Mn/CoAlOx was above 95% at 200–350 °C under a space velocity (GHSV) of 480 000 h−1. Mn dopants dramatically improved both the adsorption capacity and oxidation ability of Hg0 due to the enhancement of surface oxygen species and surface-exposed transition metal cations. Correlations between the Mn dopants, the adsorption of catalysts for Hg0 oxidation and the redox behaviors of the catalysts were established. The results revealed that two chemical reaction equilibria may have existed between adsorbed Hg0 and O2 on the surface of Mn/CoAlOx. HCl promoted the chemical reaction equilibria to the positive direction. 1Mn/CoAlOx catalysts had a certain ability to resist water and sulfur poisoning. These LDH-derived Mn/CoAlOx catalysts were first reported for Hg0 oxidation in flue gas, which could be used for practical applications.

 Please wait while we load your content...
Please wait while we load your content...