Highly selective phenol production from benzene on a platinum-loaded tungsten oxide photocatalyst with water and molecular oxygen: selective oxidation of water by holes for generating hydroxyl radical as the predominant source of the hydroxyl group†
Abstract
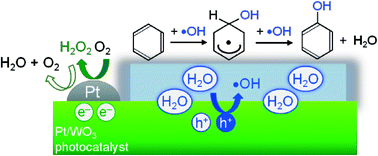
Particles of tungsten oxide loaded with nanoparticulate platinum (Pt/WO3) photocatalytically produced phenol from benzene with high selectivity (e.g., 74% at 69% of benzene conversion) in water containing molecular O2; the selectivity for phenol was much higher than that on conventional titanium oxide (TiO2) photocatalysts (both the unmodified and Pt-loaded) that generated CO2 as a main product. Results confirmed that photoexcited electrons on the Pt/WO3 photocatalysts mainly generated H2O2 from molecular O2 through a two-electron reduction; the H2O2 generated did not significantly contribute to the undesirable peroxidation of the phenol produced. In contrast, the oxygen radical species, such as ˙O2− or ˙HO2, generated on TiO2 photocatalysts partially contributed to the successive oxidation of phenol and other intermediates to reduce the selectivity for phenol. More importantly, the reactions using 18O-labeled O2 and H2O clearly revealed that the holes generated on Pt/WO3 react primarily with H2O molecules, even in the presence of benzene in aqueous solution, selectively generating ˙OH radicals that subsequently react with benzene to produce phenol. In contrast, a portion of the holes generated on TiO2 photocatalysts reacts directly with benzene molecules, which are adsorbed on the surface of TiO2 by strong interaction with surface hydroxyl groups. This direct oxidation of substances by holes undoubtedly enhanced non-selective oxidation, consequently lowering the selectivity for phenol by TiO2. The two unique features of Pt/WO3, the absence of reactive oxygen radical species from O2 and the ability to selectively oxidize water to form ˙OH, are the most likely reasons for the highly selective phenol production.

 Please wait while we load your content...
Please wait while we load your content...