Structure–activity relationships on metal-oxides: alcohol dehydration†
Abstract
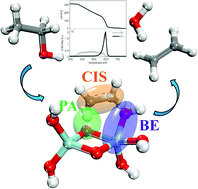
The Lewis-acid catalyzed dehydration of simple alcohols on TiO2, ZrO2 and γ-Al2O3 oxide-catalysts has been investigated by combining ab initio theoretical calculations with temperature programmed desorption (TPD) experiments. Both theoretical and experimental results demonstrate that γ-Al2O3 is more active in catalyzing the dehydration reactions than either TiO2 or ZrO2. The dehydration reaction occurs through an E2-elimination mechanism involving either surface O and/or OH groups of the oxides. Based on relationships between the dehydration barriers and key properties of the alcohols and the oxides, a dehydration model was developed that is able to screen the dehydration performance of various alcohols on different metal oxides and provide predictions that were in good agreement with the experimental dehydration barriers. The model accounts for the effect of surface hydration and the existence of surface OH-groups. The basicity of the surface oxygens is shown to be important in eliminating beta hydrogens of the alcohols. This work highlights the importance of surface OH-groups as active centers for the elimination of beta hydrogens of alcohols in Lewis-acid catalyzed dehydration reactions, a result different from the conventional view that surface OH-groups are associated with Brønsted acidity. Most importantly, a novel methodology is introduced that develops structure–activity relationships on oxides for the conversion of biomass derived molecules to chemicals.

 Please wait while we load your content...
Please wait while we load your content...