Halogen molecular modifications at high pressure: the case of iodine†
Abstract
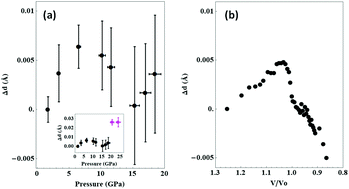
Metallization and dissociation are key transformations in diatomic molecules at high densities particularly significant for modeling giant planets. Using X-ray absorption spectroscopy and atomistic modeling, we demonstrate that in halogens, the formation of a connected molecular structure takes place at pressures well below metallization. Here we show that the iodine diatomic molecule first elongates by ∼0.007 Å up to a critical pressure of Pc ∼ 7 GPa, developing bonds between molecules. Then its length continuously decreases with pressure up to 15–20 GPa. Universal trends in halogens are shown and allow us to predict for chlorine a pressure of 42 ± 8 GPa for molecular bond-length reversal. Our findings contribute to tackling the molecule invariability paradigm in diatomic molecular phases at high pressures and may be generalized to other abundant diatomic molecules in the universe, including hydrogen.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...