Tuning of ORR activity through the stabilization of the adsorbates by hydrogen bonding with substituent groups†
Abstract
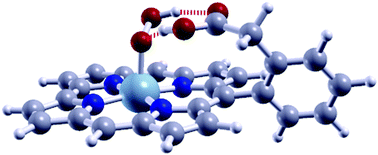
Metallocorroles and metalloporphyrins (M–N–C) are some of the best alternative molecular catalysts for the replacement of the expensive platinum-group metals (PGM) in oxygen reduction reaction (ORR) catalysis in polymer electrolyte membrane (PEM) fuel cells. To date, Co-based corroles have shown the best performance, but still suffer from the poor stability and the toxicity of the Co metal. Mn–N–C are more stable than Co–N–C, and are also less reactive towards peroxide formation. In this work, using first-principles density functional theory calculations, we study the improvement of the Mn-based corrole ORR activity by exploiting hydrogen bonding with substituent groups to modify the adsorption energies of the ORR intermediates and obtain higher onset potential (Vonset) values. We found that by using phenyl acetic acid as a substituent, Vonset increased from 0.54 V for the unsubstituted corrole to ∼0.9 V which is competitive with the Vonset of the Co-based corroles. Our results suggest that hydrogen bonding with substituent groups should be considered in the analysis and design of the reactivity of active sites in non-PGM ORR catalysts.


 Please wait while we load your content...
Please wait while we load your content...