Is non-statistical dissociation a general feature of guanine–cytosine base-pair ions? Collision-induced dissociation of a protonated 9-methylguanine–1-methylcytosine Watson–Crick base pair, and comparison with its deprotonated and radical cation analogues†
Abstract
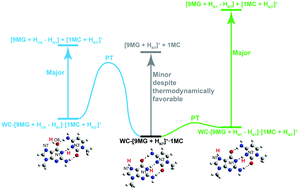
A guided-ion beam tandem mass spectrometric study was performed on collision-induced dissociation (CID) of a protonated 9-methylguanine–1-methylcytosine Watson–Crick base pair (designated as WC-[9MG·1MC + H]+), from which dissociation pathways and dissociation energies were determined. Electronic structure calculations at the DFT, RI-MP2 and DLPNO-CCSD(T) levels of theory were used to identify product structures and delineate reaction mechanisms. Intra-base-pair proton transfer (PT) of WC-[9MG·1MC + H]+ results in conventional base-pair conformations that consist of hydrogen-bonded [9MG + H]+ and 1MC and proton-transferred conformations that are formed by PT from the N1 of [9MG + H]+ to the N3′ of 1MC. Two types of conformers were distinguished by CID in which the conventional conformers produced [9MG + H]+ product ions whereas the proton-transferred conformers produced [1MC + H]+. The conventional conformers have a higher population (99.8%) and lower dissociation energy than the proton-transferred counterparts. However, in contrast to what was expected from the statistical dissociation of the equilibrium base-pair conformational ensemble, the CID product ions of WC-[9MG·1MC + H]+ were dominated by [1MC + H]+ rather than [9MG + H]+. This finding, alongside the non-statistical CID reported for deprotonated guanine–cytosine (Lu et al.; PCCP, 2016, 18, 32222) and guanine–cytosine radical cation (Sun et al.; PCCP, 2020, 22, 14875), reinforces that non-statistical dissociation is a distinctive feature of singly-charged Watson–Crick guanine–cytosine base pairs. It implies that intra-base-pair PT facilitates the formation of proton-transferred conformers in these systems and the ensuing conformers have loose transition states for dissociation. The monohydrate of WC-[9MG·1MC + H]+ preserves non-statistical CID kinetics and introduces collision-induced methanol elimination via the reaction of the water ligand with a methyl group.


 Please wait while we load your content...
Please wait while we load your content...
