Structural characterization and magnetic properties of chromium jarosite KCr3(OD)6(SO4)2†
Abstract
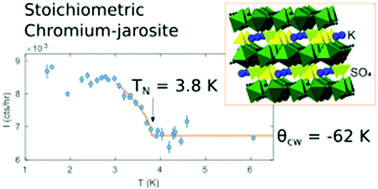
Potassium chromium jarosite, KCr3(OH)6(SO4)2 (Cr-jarosite), is considered a promising candidate to display spin liquid behavior due to the strong magnetic frustration imposed by the crystal structure. However, the ground state magnetic properties have been debated, since Cr-jarosite is notoriously non-stoichiometric. Our study reports the magnetic properties for deuterated KCr3(OD)6(SO4)2 on chemically well-defined samples, which have been characteried by a combination of powder X-ray diffraction, neutron diffraction, solid state NMR spectroscopy, and scanning electron microscopy with energy dispersive spectroscopy. Eight polycrystalline samples, which all contained only 1–3% Cr vacancies were obtained. However, significant substitution (2–27%) of potassium with H2O and/or H3O+ was observed and resulted in pronounced stacking disorder along the c-axis. A clear second-order transition to an antiferromagnetically ordered phase at TN = 3.8(1) K with a small net moment of 0.03 μB per Cr3+-ion was obtained from vibrating sample magnetometry and temperature dependent neutron diffraction. The moment is attributed to spin canting caused by the Dzyaloshinskii–Moriya interaction. Thus, our experimental results imply that even ideal potassium chromium jarosite will exhibit magnetic order below 4 K and therefore it does not qualify as a true spin liquid material.


 Please wait while we load your content...
Please wait while we load your content...