Unexpectedly strong Xe binding by host–guest interaction†
Abstract
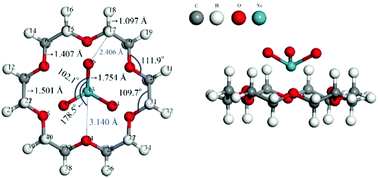
A unique noncovalent interaction between XeO3 and 18-crown-6 has been studied by density functional theory. The calculated results show that there exists an extremely strong binding force between both species, reaching 36.44 kcal mol−1, which is comparable to the strong cation–π interaction. Detailed analyses on relaxed force constants, electrostatic potentials and the independent gradient model, etc. suggest that both quite strong aerogen bondings (Xe⋯O) and relatively weak unconventional H-bondings (C–H⋯O) coexist, and the complex is a typical heterodimer with multiple binding sites. Further studies found that XeO3 takes a quick rotary motion relative to 18-crown-6 in the complex due to low rotary barrier. Another two guest molecules, KrO3 and ArO3, are also discussed.


 Please wait while we load your content...
Please wait while we load your content...