Understanding the effect of nanoconfinement on the structure of water hydrogen bond networks†
Abstract
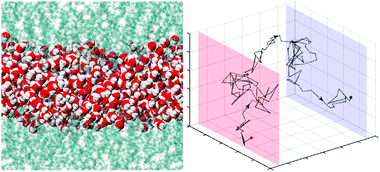
Using an integrated approach of network theory and atomistic molecular dynamics simulations, we performed a detailed topological analysis on hydrogen bond networks of water confined between either two graphene sheets or two lipid bilayers to explore the structural perturbation of these networks under nanoscale confinement. The hydrogen bond network structure can be perturbed to a considerable extent when water is confined by such surfaces, yet no small-world behaviour is observed. The presence of ions also reduces the network complexity but its effect may be small depending on the type of confining surfaces. We developed an information flow model to evaluate the fluctuating nature of hydrogen bond networks and to characterise the dynamic, long-distance hydrogen-bonded chains in water. We found that the length and directionality of the hydrogen bond “trails” are highly susceptible to the type of confining surfaces and the degree of confinement. In particular, the endpoints of the hydrogen bond trails are not completely random in confined water, in which inherent distributions are determined by the density of water and the density of hydrogen bonds. This work forms the basis for the study of the pure effect of hydrogen bond network topology on various transport processes, such as proton transfer, that occur along a sequence of hydrogen bonds in a biochemical system. Our results suggest that a combined effect of the structure and lifetime of the hydrogen bond network of interfacial water may contribute to high lateral proton diffusivity at the surface of a lipid membrane.


 Please wait while we load your content...
Please wait while we load your content...