Defects on a pyrite(100) surface produce chemical evolution of glycine under inert conditions: experimental and theoretical approaches
Abstract
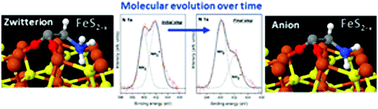
The presence of non-stoichiometric sites on the pyrite(100) surface makes it a suitable substrate for driving the chemical evolution of the amino acid glycine over time, even under inert conditions. Spectroscopic molecular fingerprints prove a transition process from a zwitterionic species to an anionic species over time on the monosulfide enriched surface. By combining experimental and theoretical approaches, we propose a surface mechanism where the interaction between the amino acid species and the surface will be driven by the quenching of the surface states at Fe sites and favoured by sulfur vacancies. This study demonstrates the potential capability of pyrite to act as a surface catalyst.


 Please wait while we load your content...
Please wait while we load your content...