Mechanisms of alumina growth via atomic layer deposition on nickel oxide and metallic nickel surfaces†
Abstract
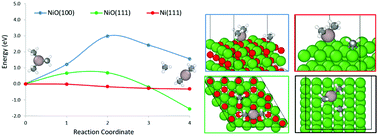
We aim at elucidating the mechanism of the trimethyl aluminum (TMA) decomposition on oxidized nickel (NiO) and metallic nickel (Ni) facets in the absence of a source of hydroxyl groups. This TMA decomposition mechanism constitutes the earliest stage of growth of Al2O3 coatings with the atomic layer decomposition (ALD) method, which stabilizes nickel catalysts in energy-intensive processes such as the dry reforming of methane. Our first-principles calculations suggest thermodynamic favorability for the TMA decomposition on metallic nickel compared to oxidized nickel. Moreover, the decomposition of TMA on metallic nickel showed almost no differences in terms of energy barriers between flat and stepped surfaces. Regarding the impact of the CH3 radicals formed after TMA decomposition, we calculated stronger adsorption on metallic nickel facets than on oxidized nickel, and these adsorption energies are comparable to the adsorption energies calculated in earlier works on Al2O3 ALD growth on palladium surfaces. These results lead us to believe in the growth of porous Al2O3 coatings triggered by CH3 contamination rather than due to preferential TMA decomposition on stepped and/or defective facets. The CH3 radicals are likely to be thermally stable at temperatures used during Al2O3 ALD processes, partially passivating the surface towards further TMA decomposition.


 Please wait while we load your content...
Please wait while we load your content...