Explicitly correlated potential energy surface of the CO2–CO van der Waals dimer and applications†
Abstract
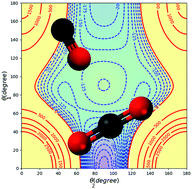
The four-dimensional-potential energy surface (4D-PES) of the CO2–CO van der Waals complex is generated using the explicitly correlated coupled cluster with single, double, and perturbative triple excitation (CCSD(T)-F12) method in conjunction with the augmented correlation-consistent triple zeta (aug-cc-pVTZ) basis set. This 4D-PES is developed over the set of inter-molecular coordinates and where the CO2 and CO monomers are treated as rigid rotors. Afterwards, analytic fits of this 4D-PES are carried out. In addition to the already known C-bound and O-bound stable structures of CO2–CO, we characterise a new isomer: it has a T-shaped structure where the O atom of the CO2 moiety points into the centre of mass of CO. We also find the saddle points connecting these minimal structures. This new isomer may play a role during the intramolecular isomerization processes at low energies. Then, the 4D-PES expansion is incorporated into bound vibrational state computations of C-bound and O-bound complexes. We also computed the temperature dependence of the second virial coefficient for CO2–CO. A good agreement with experiments is found.


 Please wait while we load your content...
Please wait while we load your content...