Superatomic nature of alkaline earth metal–water complexes: the cases of Be(H2O) 0,+4 and Mg(H2O) 0,+6†
Abstract
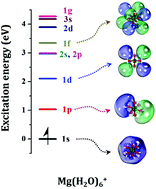
Beryllium– and magnesium–water complexes are shown to accommodate peripheral electrons around their Be2+(H2O)4 and Mg2+(H2O)6 cores in hydrogenic type orbitals. The lowest energy state of these tetra- and hexa-coordinated complexes possess one (cationic species) and two electrons (neutral species) in a pseudo spherical s-orbital, and populate p-, d-, f-, and g-type orbitals in their low-lying excited electronic states. High level quantum chemical calculations are performed to study the electronic structure of these complexes belonging to the category of solvated electrons precursors (SEPs). The observed Aufbau principle is in harmony with the previously introduced series for metal–ammonia complexes. In the current study we are able to expand the previously proposed shell model of SEPs beyond the 2d level. The observed shell model for Mg(H2O)6+ is found to be 1s, 1p, 1d, 2s, 2p, 1f, 2d, 3s, and 1g. The stability of the Be(H2O)0/+m and Mg(H2O)0/+n systems with m = 1–4 and n = 1–6 is also examined and the higher stability of metal–ammonia SEPs over metal–water SEPs is explained in terms of the metal–ligand binding energies, hydrogen bonding, and the activation energy barrier leading to H2 release.


 Please wait while we load your content...
Please wait while we load your content...