Demonstration of Baird's rule complementarity in the singlet state with implications for excited-state intramolecular proton transfer†
Abstract
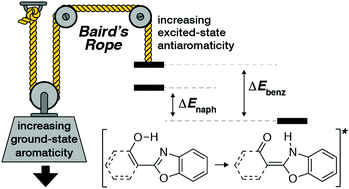
The aromatic character of an arene is proposed to switch from aromatic in the ground state (S0) to antiaromatic in the S1 and T1 excited states. This behavior is known as Baird's rule and has been invoked to explain excited-state properties, primarily in the triplet state, whereas rationalization of antiaromaticity in the singlet state is less developed. This work demonstrates the first application of Baird's rule to rationalize previously unexplained experimental behavior of the singlet state process known as excited-state intramolecular proton transfer (ESIPT). Further, by analyzing the variations in isotropic magnetic shielding around the base arenes (benzene and naphthalene) of ESIPT fluorophores in the S0 and S1 electronic states, different shielding distributions indicate a complementarity to Baird's rule: greater aromaticity in S0 leads to greater antiaromaticity in S1 and vice versa. These findings have immediate application in the design of functional ESIPT fluorophores and, more generally, for photochemical reactions that are driven by the relief of antiaromaticity in the excited state. Notably, a tenet of traditional chromophore design states that expansion of conjugation generally leads to a red-shift in absorbance and emission wavelengths. The results of this study show that ESIPT fluorophores run contrary to those conventional design principles and this behavior can only be rationalized by considering Baird's rule.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...