Impact of atmospheric water vapor on the thermal decomposition of calcium hydroxide: a universal kinetic approach to a physico-geometrical consecutive reaction in solid–gas systems under different partial pressures of product gas†
Abstract
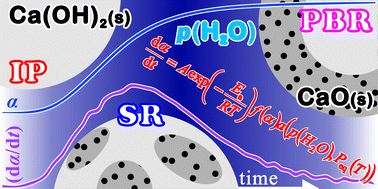
Thermal decomposition of Ca(OH)2 under atmospheric water vapor exhibits special features, including an induction period (IP) and a subsequent sigmoidal mass-loss behavior under isothermal conditions. Atmospheric water vapor reduces the reaction rate at a specific temperature and causes a systematic shift of the mass-loss curve, which was recorded at a specific heating rate, to higher temperatures as the water vapor pressure, p(H2O), increases. The challenge in this study was to universally describe the kinetics of thermal decomposition under various p(H2O) conditions by introducing an accommodation function in the fundamental kinetic equation. The accommodation function in the multiplied form of two p(H2O) components with a variable exponent in each component was derived on the basis of the classical nucleation and interface reaction theories. The universal kinetic approach was realized by applying the accommodation function to formal kinetic analyses of the Arrhenius plot for the IP and the Friedman plot for the mass-loss process. Furthermore, the overall reaction process under isothermal conditions was analyzed kinetically on the basis of the physico-geometrical consecutive reaction model, which was composed of an IP, a surface reaction (SR), and a phase boundary-controlled reaction (PBR). Subsequently, the kinetic parameters for each physico-geometrical reaction step were determined by the modified Arrhenius plot with the accommodation function. The impact of the atmospheric water vapor on the kinetics of thermal decomposition was characterized in connection with physico-geometrical reaction mechanisms through the interpretation of the kinetic parameters and these variation behavior patterns as the overall reaction advanced.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...