Spin state engineered ZnxCo3−xO4 as an efficient oxygen evolution electrocatalyst†
Abstract
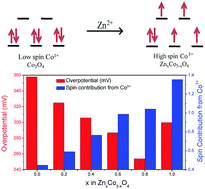
Oxygen evolution is the key step in the oxidation of water in electrolyzers and photoelectrochemical cells for the production of hydrogen. Developing a non-precious metal oxide catalyst with good electrocatalytic activity for the oxygen evolution reaction (OER) is very challenging. In this work, nanostructured ZnxCo3−xO4 has been shown as an efficient catalyst with a low overpotential for the OER in 0.1 M KOH solution. Substitution of Co2+ in the spinel oxide Co3O4 with Zn2+ creates a higher number of high-spin Co3+, which is found to be directly correlated with the OER activity of ZnxCo3−xO4. Zn0.8Co2.2O4 (x = 0.8) with the optimum amount of Co2+/Co3+ and high-spin Co3+ content showed a very low overpotential of ∼250 mV, at 10 mA cm−2, with a turnover frequency of ∼3 × 10−3 s−1 for the OER. The high Faradaic efficiency along with the stability of Zn0.8Co2.2O4 and electrocatalytic activity comparable with that of precious metal oxides indicate that this composition is a promising catalyst for water oxidation.


 Please wait while we load your content...
Please wait while we load your content...