The adverse role of excess negative ions in reducing the photoluminescence from water soluble MAA–CdSe/ZnS quantum dots in various phosphate buffers†
Abstract
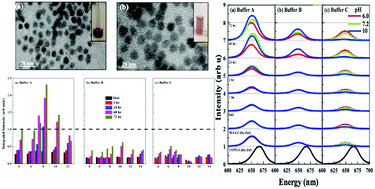
The use of CdSe/ZnS quantum dots in making biosensors or biomarkers requires them to be water soluble, which can be achieved by conjugating with MAA. We report observation of modulation in the photoluminescence intensities of MAA conjugated CdSe/ZnS QDs (MAA–QDs) that depended strongly on the types and quantity of negative ions present in various kinds of phosphate buffers. The deterioration of PL was attributed to the presence of excess ions in the media that altered the energy and occupation of HOMO and LUMO levels of MAA. Instantaneously, strong reduction in the PL intensity with pH was observed. MAA–QDs incubated for more than 24 hours in the phosphate buffer at pH ∼ 7.0–8.0 showed recovery and enhanced PL intensity, which was attributed to the presence of excess positive ions and a small amount of OH−. Saline buffers showed no significant recovery due to the presence of additional Cl− ions. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were successfully employed to determine the band edges of the MAA–QD system in the presence of excess positive or negative ions (Na+, H+, Cl−, and OH−) in the media. Thus, it is very important to have complete knowledge of the ions present in the buffer when using MAA–QDs for biomarking or biosensing applications.


 Please wait while we load your content...
Please wait while we load your content...